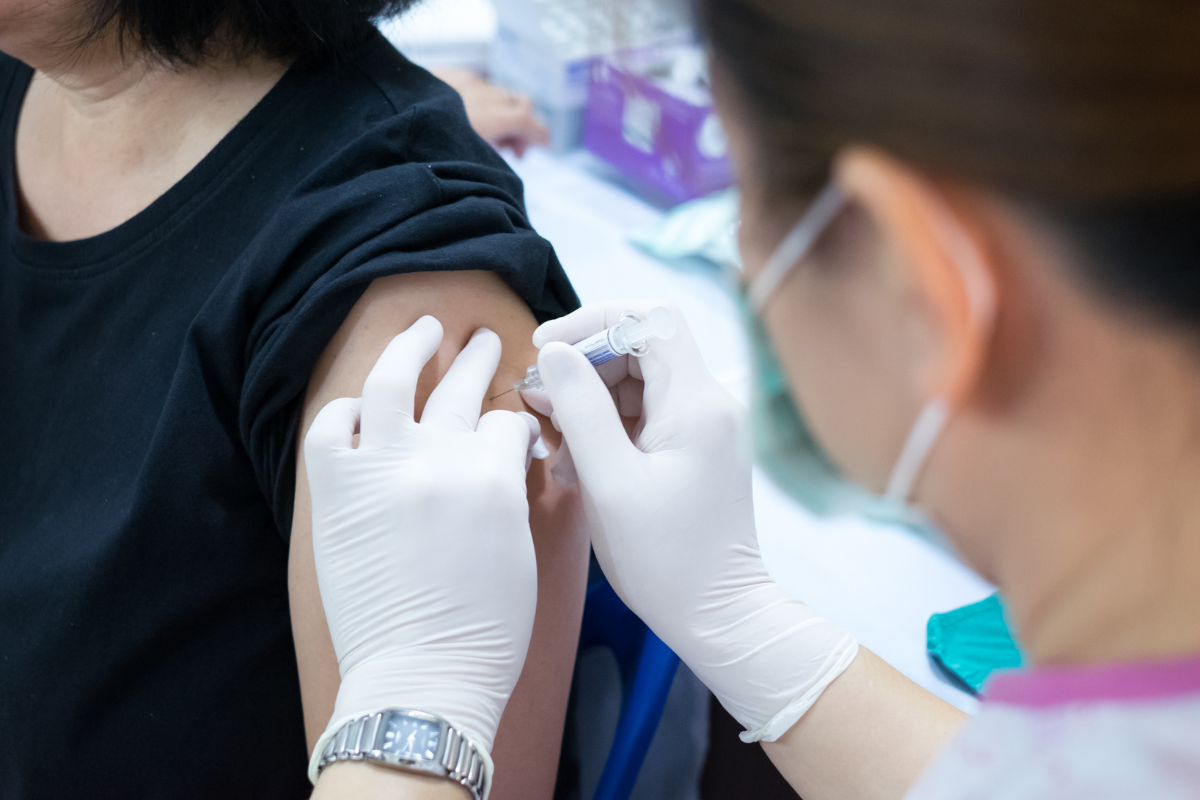
The U.S. plans to probe why some people experience allergic reactions from Pfizer's coronavirus vaccine shots.
Alkis Togias, chief of the National Institute of Allergy and Infectious Diseases' Allergy, Asthma, and Airway Biology Branch, told CNBC that the study will include “several hundred” people who have a history of severe allergic reactions.
His unit will handle the study, which may start in a matter of weeks though the schedule is not definite. The allergic reactions from Pfizer were reported by people who took a dose already.
Togias explained that NIAID researchers wanted to examine the issue after reports that a few people experienced reactions to Pfizer’s vaccine that qualified as anaphylaxis.
For instance, a clinician in Alaska manifested anaphylactic symptoms about 10 minutes after taking Pfizer’s vaccine, becoming the third health-care worker in the state to have an adverse reaction to the new treatment.
“We are a little bit concerned that people who have had a lot of allergies who have had reactions like this to all kinds of things, not just vaccines, may be afraid to get vaccinated now,” Togias said. “We just don’t want that to happen. We want to find a way for them to get vaccinated,” he added.
Allergic reactions from Pfizer, immune mechanisms
President Donald Trump’s coronavirus vaccine czar, Moncef Slaoui, also cited the study at an Operation Warp Speed briefing.
“There is now advanced planning for a study in highly allergic individuals in clinical trials to test the Moderna and Pfizer vaccines and try to understand the immune mechanisms that are underpinning any reactions,” he said.
The plan comes as the U.S. government starts shipping nearly 8 million doses of Covid vaccine across the country this week after delivering 2.9 million doses of Pfizer’s vaccine last week.
The U.S. is releasing 5.9 million doses of Moderna’s vaccine as well as 2 million doses of Pfizer’s vaccine this week, according to Health and Human Services Secretary Alex Azar. As of Sunday, 556,208 Americans have already taken the shots, according to the Centers for Disease Control and Prevention.
Doran Fink, deputy director of FDA’s division of vaccines and related products applications, announced that the agency will determine whether additional recommendations on the vaccines will be required after the investigation.
“At this point, we don’t have enough data to make a definitive recommendation one way or the other," he said during a meeting of the Vaccines and Related Biological Products Advisory Committee.
Togias hopes the NIAID study will come up with information on the allergic reactions. He added that the study may involve people who do not manifest allergic reactions so researchers can make comparisons.
The agency will also release a comprehensive protocol that will need to be approved by the FDA before the study proceeds, Togias said. After it gets approval from the FDA, it will then need to be evaluated and approved by an ethics committee.
“Of course, everybody when they hear a study that relates to the vaccine, we try to be sensitive and move fast,” he said. “But it is not something we can design today and start tomorrow.”






